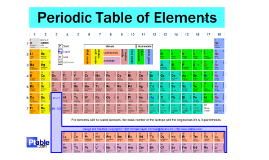
Metals and Nonmetals
Transcript: Non-Metals include elements such as Oxygen, Fluorine, and Sulfur. Non-Metals also include Metalloids, which share properties of both Metals, and non-metals, such as Arsenic, Silicon, and Germanium. Chemical Properties include the ability to gain or "lose" electrons in a process called "Bonding," have 4-8 electrons on their outer shell, and create Acidic oxidized agents. Non-Metals have higher electronegativities and are good reducing agents. Non-Metals can take on any shape or definition, depending on the temperature, and what element it is. They are brittle if solid, poor conductors, not ductile, and are transparent. Metals consist of elements like Iron, Lead, and Aluminum. Metals usually have 1-2 valence electrons in their outer shells. They react with nonmetals and create basic oxidized agents. They are good conductors and have a low electronegativity. All metals are solid at room temperature, except for Hg. Physical properties include the ability to conduct electricity, Malleable, Ductile, and Opaque like a sheet. Gerald E. Greiner III Hribek 7th 4-9-13 METAL METAL Non-Metals are found on the right side of the periodic table, along with the metalloids. The most reactive and Non-reactive group is called the Noble Gasses, which only react with themselves, and no other element. These elements can be very, very dense, and are grouped together again, by how they react, and act. Metals and Non-metals are located on the Periodic table and have varying electrons, protons, neutrons which put them in groups, and families, depending on how they react with one another, and what they do on their own. Metals are located on the left side of the periodic table, and their most reactive group is called the Alkali Metals. These metals react violently with other elements, and can be very unstable. METAL NONMETAL Metals and Non-Metals NONMETAL